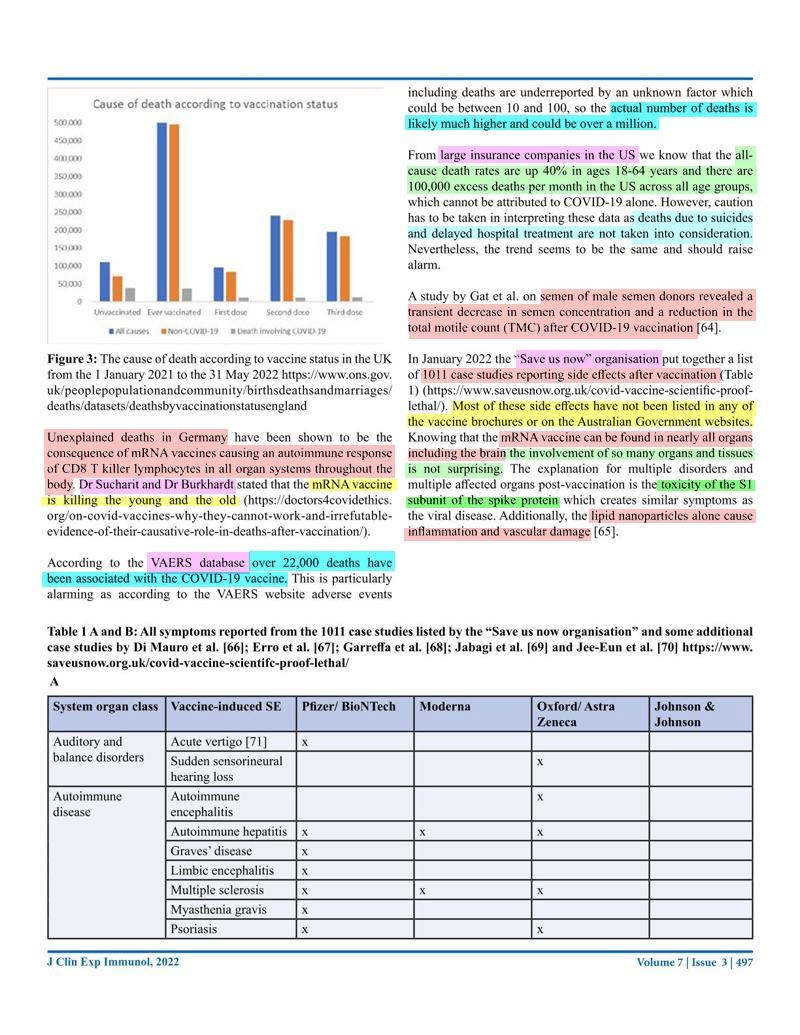
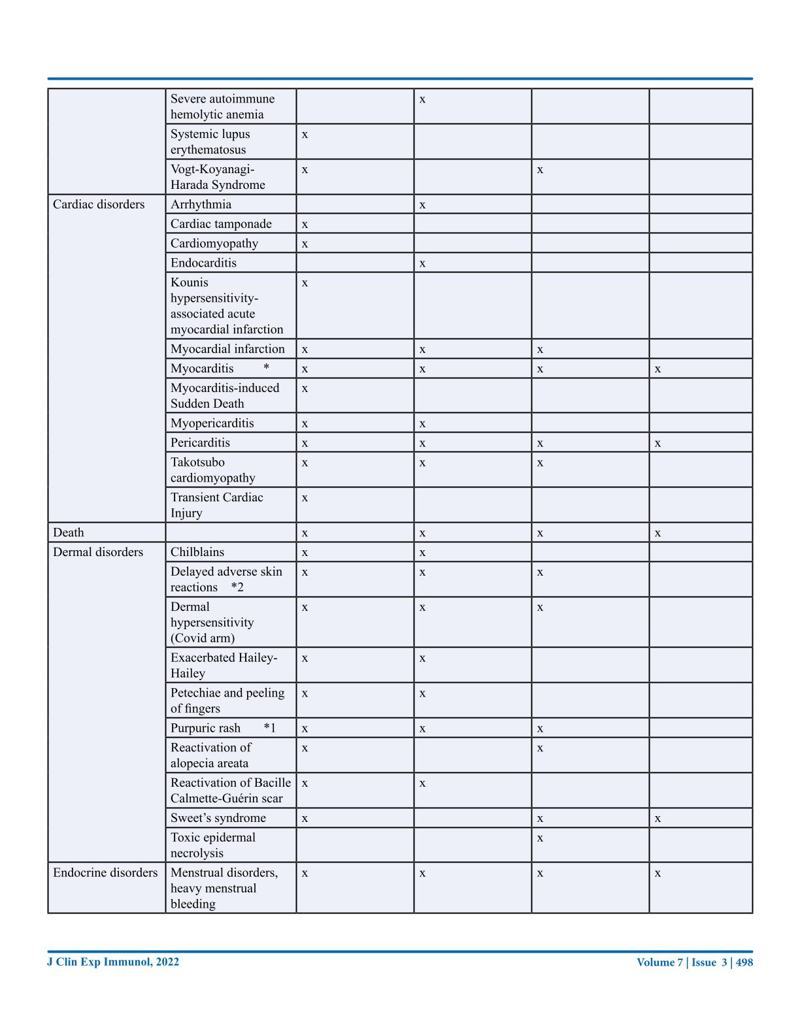
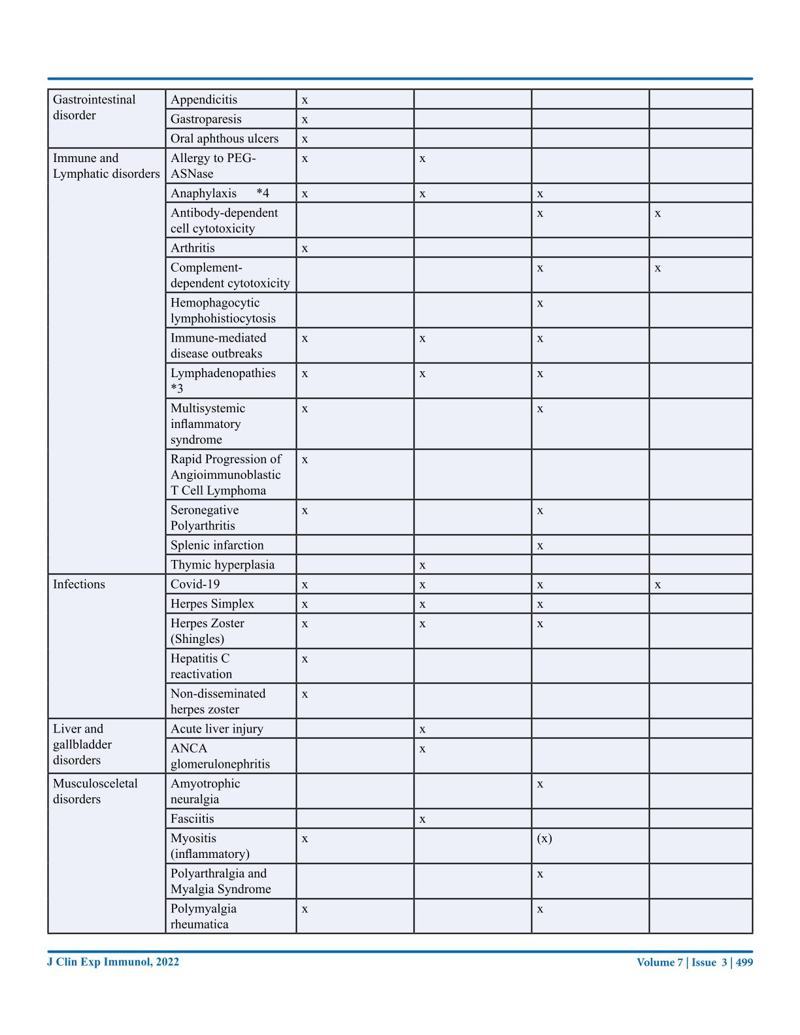
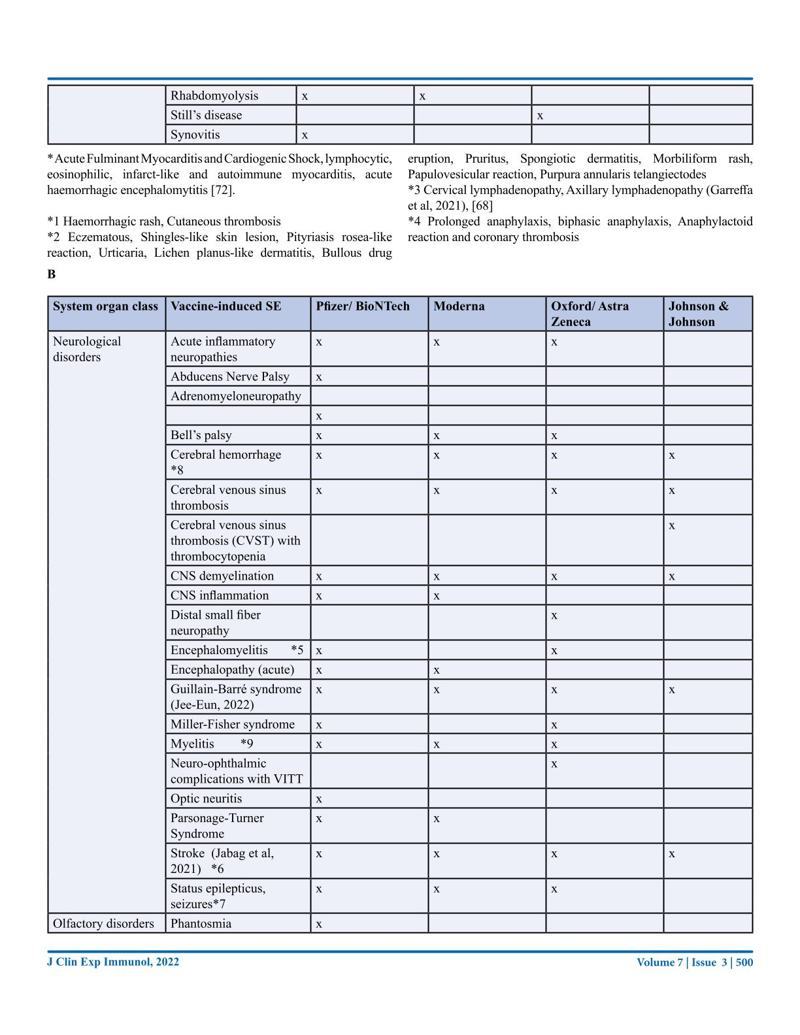
Journal of Clinical & Experimental Immunology, Published: 21 Sep 2022. New Australian paper calls out the severe side effects and false covid information from government agencies.
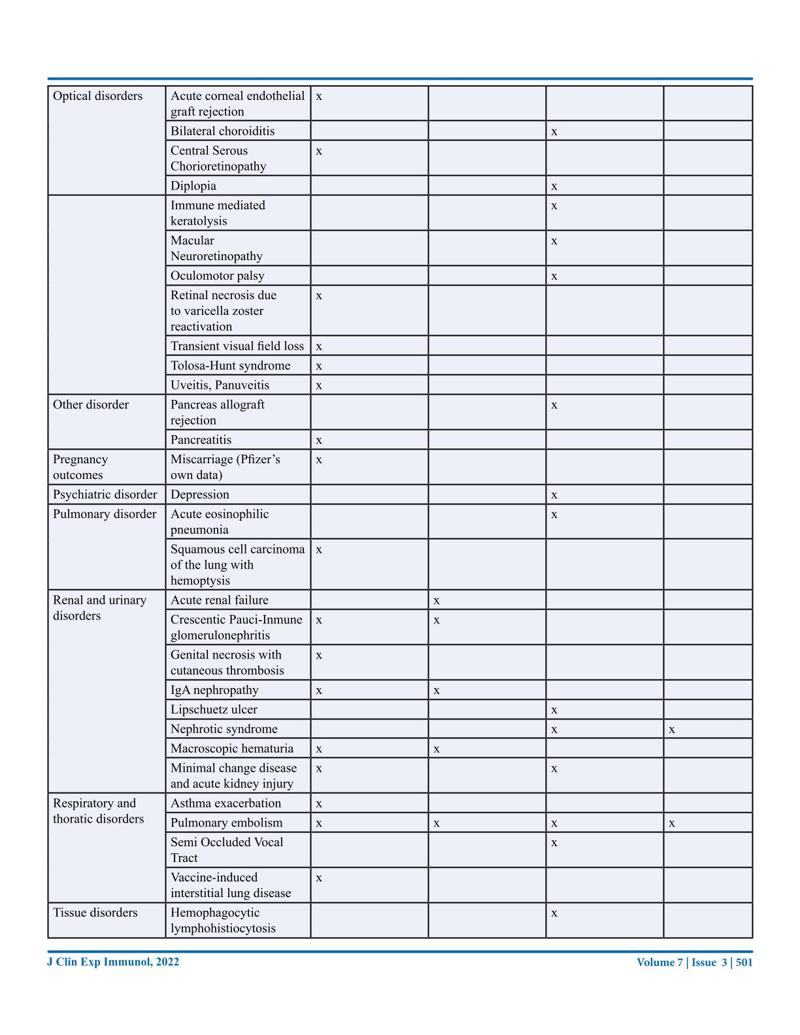
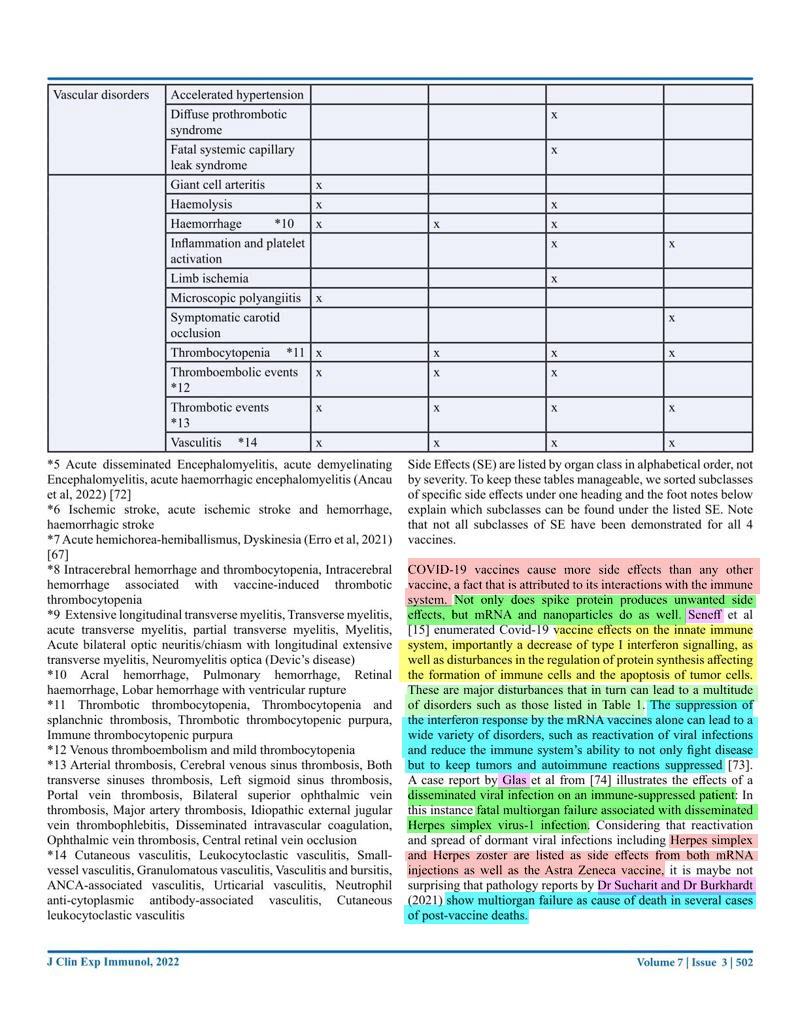
The effects of these vaccinations are slowly becoming apparent after millions of people have been vaccinated as often as four times within a year. This review has been written from an Australian perspective with the main focus on the COVID-19 mRNA vaccines. We will look at the promises and predictions originally made and the actual facts. We will evaluate the safety and efficacy by looking at the literature and the data from government agencies. The literature review will be summed up in a table listing the so far reported side effects, many of which are very serious, including death, with this data coming from 1011 case reports. Long-term side effects will also be covered, and the risk-benefit ratio will be explored. The review is ending with some very critical questions that need further discussion.
COVID-19 vaccines – An Australian Review | Journal of Clinical & Experimental Immunology | Conny Turni, Astrid Lefringhausen | Submitted: 10 Sep 2022; Accepted: 12 Sep 2022; Published: 21 Sep 2022
I’ve just gone through and highlighted the whole paper, if you want to browse by image whilst waiting for the summary – click on an image and scroll through with the arrows. It might be hard to read given that it doesn’t go fullscreen on computers, so I’ll also upload the images to my Telegram channel if you want to download or read them there.
ISSN: 2475-6296
Queensland Alliance for Agriculture and Food Innovation, the University of Queensland, St Lucia, Queensland 4067, Australia.
References/Footnotes:
- 1. Hansen, T., Titze, U., Kulamadayil-Heidenreich, N. S. A., Glombitza, S., Tebbe, J. J., Röcken, C., & Wilkens, L. (2021). First case of postmortem study in a patient vaccinated against SARS-CoV-2. International Journal of Infectious Diseases, 107, 172-175. ↥
- 2. Ndeupen, S., Qin, Z., Jacobsen, S., Bouteau, A., Estanbouli, H., & Igyártó, B. Z. (2021). The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. Iscience, 24(12), 103479. ↥
- 3. Zhou, Y., Peng, Z., Seven, E. S., & Leblanc, R. M. (2018). Crossing the blood-brain barrier with nanoparticles. Journal of controlled release, 270, 290-303. ↥
- 4. Wick, P., Malek, A., Manser, P., Meili, D., Maeder-Althaus,X., Diener, L., … & von Mandach, U. (2010). Barrier capacity of human placenta for nanosized materials. Environmental health perspectives, 118(3), 432-436. ↥
- 5. Shimazawa, R., & Ikeda, M. (2021). Potential adverse events in Japanese women who received tozinameran (BNT162b2, Pfizer-BioNTech). Journal of Pharmaceutical Policy and Practice, 14(1), 1-3. ↥
- 6. Karikó, K., Buckstein, M., Ni, H., & Weissman, D. (2005). Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity, 23(2), 165-175. ↥
- 7. Karikó, K., Muramatsu, H., Welsh, F. A., Ludwig, J., Kato, H., Akira, S., & Weissman, D. (2008). Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Molecular therapy, 16(11), 1833-1840. ↥
- 8. Röltgen, K., Nielsen, S.C.A., Silv,a O., Younes, S.F. et al. (2022). Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell, 185, 1025 – 1040. DOI: https://doi.org/10.1016/j.cell.2022.01.018 ↥
- 9. Biancatelli, R. M. C., Solopov, P. A., Sharlow, E. R., Lazo, J. S., Marik, P. E., & Catravas, J. D. (2021). The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in ?18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology. ↥
- 10. Zhang, S., Liu, Y., Wang, X., Yang, L., Li, H., Wang, Y., … & Hu, L. (2020). SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. Journal of hematology & oncology, 13(1), 1-22. ↥
- 11. Cattin-Ortolá, J., Welch, L. G., Maslen, S. L., Papa, G., James, L. C., & Munro, S. (2021). Sequences in the cytoplasmic tail of SARS-CoV-2 Spike facilitate expression at the cell surface and syncytia formation. Nature communications, 12(1), 1-11. ↥
- 12. Cheng, Y. W., Chao, T. L., Li, C. L., Wang, S. H., Kao, H. C., Tsai, Y. M., … & Yeh, S. H. (2021). D614G substitution of SARS-CoV-2 spike protein increases syncytium formation and virus titer via enhanced furin-mediated spike cleavage. Mbio, 12(4), e00587-21. ↥
- 13. Singh, N., & Singh, A. B. (2020). S2 subunit of SARS-nCoV-2 interacts with tumor suppressor protein p53 and BRCA: an in silico study. Translational Oncology, 13(10), 100814. ↥
- 73. Passegu, E., Ernst, P.A. (2009). IFN-alpha wakes up sleeping hematopoietic stem cells Natural Medicine, 15 (6), Article 612613, 10.1038/nm0609-612 ↥↥
- 14. Liu, J., Wang, J., Xu, J., Xia, H., Wang, Y., Zhang, C., …& Liu, Z. (2021). Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines. Cell discovery, 7(1), 1-15. ↥
- 15. Seneff, S., Nigh, G., Kyriakopoulos, A. M., & McCullough, P. A. (2022). Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food and Chemical Toxicology, 164, 113008. ↥↥
- 16. Mishra, R., Banerjea, A.C. (2021) SARS-CoV-2 Spike Targets USP33-IRF9 Axis via Exosomal miR-148a to Activate Human Microglia. Frontiers in Immunology, 12. DOI=10.3389/fimmu.2021.656700 ↥
- 17. Reiss, K., & Bhakdi, S. (2020). Corona, False Alarm?: Facts and Figures. Chelsea Green Publishing. ↥
- 18. Doshi, P. (2020). Covid-19: Do many people have pre-existing immunity?. Bmj, 370. ↥
- 19. Ng, K. W., Faulkner, N., Cornish, G. H., Rosa, A., Harvey, R., Hussain, S., … & Kassiotis, G. (2020). Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science, 370(6522), 1339-1343. ↥
- 20. King, E. M. (2020). T-cells Are the Superstars in Fighting COVID-19. But Why are some People So Poor at Making Them?. bmj, 370. ↥
- 21. Phillips, N. (2021). The corona virus will become endemic. Nature, 590: 382-384. ↥
- 22, 10791. doi: 10.3390/ijms221910791. ↥
- 23. Oran, D.P., Topol, E.J. (2021). The Proportion of SARS-CoV-2 Infections That Are Asymptomatic : A Systematic Review. Annual Interntional Medicines, 174(5): 655-662. doi: 10.7326/M20-6976. ↥
- 24. Ivanova, E., Devlin, J., Buus, T., Koide, A., Cornelius, A., Samanovic, M., … & Koralov, S. B. (2021). Discrete immune response signature to SARS-CoV-2 mRNA vaccination versus infection. ↥
- 25. Seneff, S. and Nigh, G. (2021). Worse than the disease? Reviewing some possible unintended consequences of the mRNA vaccines against COVID-19. International Journal of Vaccine Theory, Practice, and Research, 2, 38 – 79. ↥
- 26. Cardozo, T., & Veazey, R. (2021). Informed consent disclosure to vaccine trial subjects of risk of COVID-19 vaccines worsening clinical disease. International journal of clinical practice, 75(3), e13795. ↥
- 27. Nordström, P., Ballin, M., & Nordström, A. (2022). Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. The Lancet Infectious Diseases, 22(6), 781-790. ↥
- 28. Nordström, P., Ballin, M., & Nordström, A. (2022). Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden. The Lancet, 399(10327), 814-823. ↥
- 29. Israel, A., Shenhar, Y., Green, I., Merzon, E., Golan-Cohen, A., Schäffer, A. A., … & Magen, E. (2021). Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Vaccines, 10(1), 64. ↥
- 30. Lozano-Ojalvo, D., Camara, C., Lopez-Granados, E., Nozal, P., del Pino-Molina, L., Bravo-Gallego, L. Y., … & Ochando, J. (2021). Differential effects of the second SARS-CoV-2 mRNA vaccine dose on T cell immunity in naive and COVID-19 recovered individuals. Cell reports, 36(8), 109570. ↥
- 31. Keehner, J., Binkin, N.J., Laurent, L.C., Pride, D. (2021). Resurgence of SARS-CoV-2 Infection in a Highly Vaccinated Health System Workforce. The New England Journal of Medicine, 385, 1330-1332. DOI: 10.1056/NEJMc2112981. ↥
- 32. Chau, N. V. V., Ngoc, N. M., Nguyet, L. A., Quang, V. M., Ny, N. T. H., Khoa, D. B., … & OUCRU COVID-19 research group. (2021). An observational study of breakthrough SARSCoV-2 Delta variant infections among vaccinated healthcare workers in Vietnam. EClinicalMedicine, 41, 101143. ↥
- 33. Hetemäki, I., Kääriäinen, S., Alho, P., Mikkola, J., Savolainen-Kopra, C., Ikonen, N., Nohynek, H., Lyytikäinen, O. (2021). An outbreak caused by the SARS-CoV-2 Delta variant (B.1.617.2) in a secondary care hospital in Finland, May 2021. Eurosurveillance, 26, 2100636 (2021), https://doi.org/10.2807/1560-7917.ES.2021.26.30.2100636. ↥
- 34. Shitrit, P., Zuckerman, N. S., Mor, O., Gottesman, B. S., & Chowers, M. (2021). Nosocomial outbreak caused by the SARS-CoV-2 Delta variant in a highly vaccinated population, Israel, July 2021. Eurosurveillance, 26(39), 2100822. ↥
- 35. Acharya, C. B., Schrom, J., Mitchell, A. M., Coil, D. A., Marquez, C., Rojas, S., … & Havlir, D. (2021). No significant difference in viral load between vaccinated and unvaccinated, asymptomatic and symptomatic groups infected with SARSCoV-2 delta variant. MedRxiv. ↥
- 36. Riemersma, K. K., Grogan, B. E., Kita-Yarbro, A., Jeppson, G. E., O’Connor, D. H., Friedrich, T. C., & Grande, K. M. (2021). Vaccinated and unvaccinated individuals have similar viral loads in communities with a high prevalence of the SARS-CoV-2 delta variant. MedRxiv, 2021-07. ↥
- 37. Brown C.M., Vostok J., Johnson H., Burns, M., Gharpure, R., Sami, S., Sabo, R.T., Hall, N., Foreman, A., Schubert, P.L., Gallagher, G.R., Fink, T., Madoff, L.C., Gabriel, S.B.,MacInnis, B., Park, D.J., Siddle, K.J., Harik, V., Arvidson, D Brock-Fisher, T., Dunn, M., Kearns, A., Laney, A.S., 2021. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings – Barnstable County, Massachusetts, July 2021. MMWR Morbidity and Mortality Weekly Reports 70, 1059- 1062. ↥
- 38. Servellita, V., Morris, M. K., Sotomayor-Gonzalez, A., Gliwa, A. S., Torres, E., Brazer, N., … & Chiu, C. Y. (2022). Predominance of antibody-resistant SARS-CoV-2 variants in vaccine breakthrough cases from the San Francisco Bay Area, California. Nature microbiology, 7(2), 277-288. ↥
- 39. Subramanian, S. V., & Kumar, A. (2021). Increases in COVID-19 are unrelated to levels of vaccination across 68 countries and 2947 counties in the United States. European journal of epidemiology, 36(12), 1237-1240. ↥
- 40. Kirsch, S. (2021) “New Studies Show that the COVID Vaccines Damage your Immune System, Likely Permanently,” Steve Kirsch’s Newsletter, Dec. 24, 2021, https://stevekirsch.substack.com/p/new-stu-dy-shows-vaccines-must-be. ↥
- 41. Kirsch, S. (2022a). “Pfizer CEO says Two Covid Vaccine Doses Aren’t Enough for Omicron,” Steve Kirsch’s Newsletter Jan. 10, 2022, https://stevekirsch.subs-tack.com/p/pfizer-ceo-saystwo-covid-vaccine. ↥
- 42. 36. Ioannidis, J. P. (2021). Infection fatality rate of COVID-19 inferred from seroprevalence data. Bulletin of the World Health Organization, 99(1), 19. ↥
- 43. Beattie, K.A. (2021) Worldwide Bayesian Causal Impact Analysis of Vaccine Administration on Deaths and Cases Associated with COVID-19: A BigData Analysis of 145 Countries. Department of Political Science University of Alberta Alberta, Canada. ↥
- 65. Psichogiou, M., Karabinis, A., Poulakou, G., Antoniadou, A., Kotanidou, A., Degiannis, D., … & Hatzakis, A. (2021). Comparative immunogenicity of BNT162B2 mRNA vaccine with natural covid-19 infection. medRxiv. ↥
- 44. Reuters, “EU Drug Regulator Expresses Doubt on Need for Fourth Booster Dose,” Jan. 11, 2022, https://www.reuters.com/business/healthcare-pharmaceuticals/eu-drug-regulator-saysmore-data–needed-impact-omicron-vaccines-2022-01-11/. ↥
- 45. Kirsch, S. (2022b). “Top Israeli Immunologist Criticizes Pandemic Response in Open Letter,” Jan 13, 2022, Steve Kirsch’s Newsletter, https://stevekirsch.substack.com/p/top-israeli-immunologist–criticizes?r=15hae6&utm_campaign=post&utm_medium=email. ↥
- 46. Thorp, J.A., Renz, T., Northrup, C., et al. (2022). Patient betrayal: The Corruption of healthcare, informed consent and the physician – patient relationship. 0. https://www.doi.org/10.46766/tjegms. ↥
- 47. Doshi, P. 2020. Covid-19: Do many people have pre-existing immunity? 17 September 2020 BMJ 2020; 370 doi: https://doi.org/10.1136/bmj.m3563 **** I believe the authors mis-referenced the study, I believe the study they meant to reference was this one ↥
- 48. Shimabukuro, T. T., Kim, S. Y., Myers, T. R., Moro, P. L Oduyebo, T., Panagiotakopoulos, L., … & Meaney-Delman, D. M. (2021). Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. New England Journal of Medicine. ↥
- 83. Anand, P., & Stahel, V. P. (2021). The safety of Covid-19 mRNA vaccines: A review. Patient safety in surgery, 15(1), 1-9. ↥↥↥
- 89. Walsh, E.E., Frenck, R.W., Falsey, A.R. Jr., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., Neuzil, K., Mulligan, M.J., Bailey, R. (2020). Safety and immunogenicity of two RNA-based Covid–19 vaccine candidates. New England Journal of Medicine, 383, 2439–50. ↥
- 90. Saito, S., Nakashima, A., Shima, T., & Ito, M. (2010). Th1/ Th2/Th17 and regulatory T?cell paradigm in pregnancy. American journal of reproductive immunology, 63(6), 601- 610. ↥
- 91. Helmo, F. R., Alves, E. A. R., Moreira, R. A. D. A., Severino, V. O., Rocha, L. P., Monteiro, M. L. G. D. R., … & Corrêa, R. R. M. (2018). Intrauterine infection, immune system and premature birth. The journal of maternal-fetal & neonatal medicine, 31(9), 1227-1233. ↥
- 49. Regev-Yochay, G., Gonen, T., Gilboa, M., Mandelboim, M., Indenbaum, V., Amit, S., … & Lustig, Y. (2022). Efficacy of a fourth dose of COVID-19 mRNA vaccine against omicron. New England Journal of Medicine, 386(14), 1377-1380. ↥
- 50. Regev-Yochay, G., Gonen, T., Gilboa, M., Mandelboim, M., Indenbaum, V., Amit, S., … & Lustig, Y. 4th Dose COVID mRNA Vaccines’ Immunogenicity & Efficacy Against Omicron VOC (preprint). ↥
- 51. Dopp, K, Seneff, S. (2022). COVID-19 and All-Cause Mortality Data by Age Group Reveals Risk of COVID Vaccine-Induced Fatality is Equal to or Greater than the Risk of a COVID death for all Age Groups Under 80 Years Oldas 2022: 1-21. ↥
- 52. Kostoff, R.N., Calina, D., Kanduc, D., Briggs, M.B., Vlachoyiannopoulos, P., Svistunov, A.A., Tsatsakis, A.. 2021. Why are we vaccinating children against COVID-19? Toxicology Reports. 8, 1665-1684. ↥
- 53. Goldberg, Y., Mandel, M., Bar-On, Y.M., Bodenheimer, O.,Freedman, L., Haas, E.J., Milo, R., Alroy-Preis, S., Ash, N., Huppert, A. (2021). Waning Immunity after the BNT162b2 Vaccine in Israel. New England Journal of Medicine, 385(24):e85. doi: 10.1056/NEJMoa2114228. ↥
- 54. Jo, D.-H., Minn, D., Lim, J., Lee, K.-D., Kang, Y.-M., Choe, K.-W., Kim, K.-N. (2021). Rapidly declining SARSCoV-2 antibody titers within 4 months after BNT162b2 vaccination. Vaccines, 9, 1145. ↥
- 55. Israel, A., Shenhar, Y., Green, I., Merzon, E., Golan- Cohen, A., Schäffer, A.A., Ruppin, E., Vinke,r S, Magen, E. (2021). Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. medRxiv [Preprint]. 2021 Aug 21:2021.08.19.21262111. doi: 10.1101/2021.08.19.21262111. Update in: Vaccines (Basel). 2021 Dec 31;10(1): PMID: 34462761; PMCID: PMC8404903. ↥
- 56. Binkin, N.J., Laurent, L.C., Pride, D., Longhurst, C.A., Abeles, S.R., Torriani, F.J. (2021). Resurgence of SARS-CoV-2 infection in highly vaccinated health system workforce. The New England Journal of Medicine. https://www.nejm.org/doi/full/10.1056/NEJMc2112981 ↥
- 57. Cromer, D., Steain, M., Reynaldi, A., Schlub, T.E., Wheatley, A.K., Juno, J.A., Kent, S.J., Triccas, J.A., Khoury, D.S., Davenport, M,P. (2022). Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. The Lancet Microbe 3, E52-E61 ↥
- 58. Krause P.R., Fleming, T.R.., Peto, R., Longini, I., Figueroa, J.P., Sterne, J.A.C., et al. (2021). Considerations in boosting COVID-19 vaccine immune responses. The Lancet 398, 1377 -10380. ↥
- 59. Lui, L., Luo, Y., Chu, H. et al. (2021) Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature https://doi.org/10.1038/d41586-021-03826-3(2021). ↥
- 60. Hachmann, N. P., Miller, J., Collier, A. R. Y., Ventura, J. D., Yu, J., Rowe, M., … & Barouch, D. H. (2022). Neutralization escape by SARS-CoV-2 Omicron subvariants BA. 2.12. 1, BA. 4, and BA. 5. New England Journal of Medicine, 387(1), 86-88. ↥
- 61. Yamamoto, K. (2022). Adverse effects of COVID-19 vaccines and measures to prevent them. Virology Journal, 19(1), 1-3. ↥
- 62. Bryant, A., Lawrie, T. A., Dowswell, T., Fordham, E. J., Mitchell, S., Hill, S. R., & Tham, T. C. (2021). Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. American journal of therapeutics, 28(4), e434. ↥
- 63. Bruno, R., Mccullough, P. A., Vila, T. F. I., Henrion-Caude, A., Garcia-Gasca, T., Zaitzeva, G. P., … & Acevedo-Whitehouse, K. (2021). SARS-CoV-2 mass vaccination: Urgent questions on vaccine safety that demand answers from international health agencies, regulatory authorities, governments and vaccine developers. Authorea Preprints. ↥
- 46. Thorp, J.A., Renz, T., Northrup, C., et al. (2022). Patient betrayal: The Corruption of healthcare, informed consent and the physician – patient relationship. 0. https://www.doi.org/10.46766/tjegms. ↥
- 64. Gat, I., Kedem, A., Dviri, M., Umanski, A., Levi, M., Hourvitz, A., & Baum, M. (2022). Covid?19 vaccination BNT162b2 temporarily impairs semen concentration and total motile count among semen donors. Andrology, 10(6), 1016-1022. ↥
- 75. Salamanna, F., Maglio, M., Landini, M.P., Fini, M. (2020). Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2. Frontiers in Medicine, 7, https://www.frontiersin.org/articles/10.3389/fmed.2020.594495 ↥
- 74. Glas, M., Smola, S., Pfuhl, T., Pokorny, J., Bohle, R.M., Bücker, A., Kamradt J., Volk T. Fatal Multiorgan Failure Associated with Disseminated Herpes Simplex Virus-1 Infection: A Case Report. Case Reports in Critical Care, Volume 2012 doi: 10.1155/2012/359360 ↥
- 76. Jocher, G., Grass, V., Tschirner, S. K., Riepler, L., Breimann, S., Kaya, T., … & Lichtenthaler, S. F. (2022). ADAM10 and ADAM17 promote SARS?CoV?2 cell entry and spike proteinmediated lung cell fusion. EMBO reports, 23(6), e54305. ↥
- 77. Pepe, A., Pietropaoli, S., Vos, M., Barba-Spaeth, G., & Zurzolo, C. (2022). Tunneling nanotubes provide a route for SARS-CoV-2 spreading. Science advances, 8(29), eabo0171. ↥↥
- 78. Kelleni, M. T. (2021). SARS CoV-2 Vaccination Autoimmunity, Antibody Dependent Covid-19 Enhancement and Other Potential Risks: Beneath the Tip of the Iceberg. International Journal of Pulmonary & Respiratory Sciences, 5(2), 555658. ↥↥
- 79. Lyons-Weiler, J. (2020). Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. Journal of translational autoimmunity, 3, 100051. ↥↥
- 80. Hasan, A., Al-Mulla, M. R., Abubaker, J., & Al-Mulla, F. (2021). Early insight into antibody-dependent enhancement after SARS-CoV-2 mRNA vaccination. Human Vaccines & Immunotherapeutics, 17(11), 4121-4125. ↥↥
- 81. Classen, J. B. (2021). COVID-19 RNA based vaccines and the risk of prion disease. Microbiol Infect Dis, 5(1), 1-3. ↥↥
- 82. Idrees, D., & Kumar, V. (2021). SARS-CoV-2 spike protein interactions with amyloidogenic proteins: Potential clues to neurodegeneration. Biochemical and biophysical research communications, 554, 94-98. ↥↥
- 84. Aldén, M., Olofsson Falla, F., Yang, D., Barghouth, M., Luan, C., Rasmussen, M., & De Marinis, Y. (2022). Intracellular reverse transcription of Pfizer BioNTech COVID-19 mRNA vaccine BNT162b2 in vitro in human liver cell line. Current issues in molecular biology, 44(3), 1115-1126. ↥
- 86. Finisterer J., Scorza, F.A. 2021. SARS-CoV-2 vaccines are not free of neurological side effects. Acta Neurology Scandinavia 144, 109-110. doi: 10.1111/ane.13451 ↥
- 87. Kuvandik, A., Özcan, E., Serin, S., Sungurtekin, H. (2021). Creutzfeldt-Jakob Disease After the COVID-19 Vaccination. Turkish Journal of Intensive Care. DOI: 10.4274/tybd.galenos.2021.91885. ↥
- 88. Serin, S., & Sungurtekin, H. (2021). Creutzfeldt-Jakob Disease After the COVID-19 Vaccination. ↥
- 22. Kircheis, R. 2021. Coagulopathies after vaccination against SARS-CoV-2 may be derived from a combined effect of SARS-CoV-2 spike protein and adenovirus vector-triggered signaling pathways. International Journal of Molecular Science, ↥
- 92. Gundry, S. R. (2021). MRNA COVID vaccines dramatically increase endothelial inflammatory markers and ACS risk as measured by the PULS cardiac test: A warning. Circulation. ↥
- 93. Bruno, R., Mccullough, P.A., Forcades, I., Vila, T. et al. (2021). SARS-CoV-2 mass vaccination: Urgent questions on vaccine safety that demand answers from international health agencies, regulatory authorities, governments and vaccine developers. Authorea, May 24, 2021. DOI: 10.22541/ au.162136772.22862058/v2. ↥↥
- 94. Blaylock, R. L. (2022). COVID UPDATE: What is the truth?.Surgical Neurology International, 13. ↥
- Conny Turni and Astrid Lefringhausen (2022) COVID-19 vaccines – An Australian Review. Journal of Clinical & Experimental Immunology. 7(3):491-508 21 Sep 2022 https://opastpublishers.com/open-access/covid-19-vaccines-an-australian-review.pdf ↥
Site Notifications/Chat:
- Telegram Post Updates @JourneyToABetterLife (channel)
- Telegram Chatroom @JourneyBetterLifeCHAT (say hi / share info)
Videos: